Pipeline | Our Process | Nutent Therapeutics
PROBLEMS
2. Mislabeled or adulterated product content of herbal dietary supplements and nutraceuticals have led to product removal in several states.
SOLUTION
1. Cyclodextrin has been used to improve curcumin’s delivery and bioavailability via its encapsulation.
2. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake.
VOLT03
VOLT03 is the only pharmaceutical-grade complexed

SUPERIOR PRECLINICAL DATA
The efficacy of VOLT03 was compared to native curcumin, indomethacin and placebo in a carrageenan mouse paw edema model.
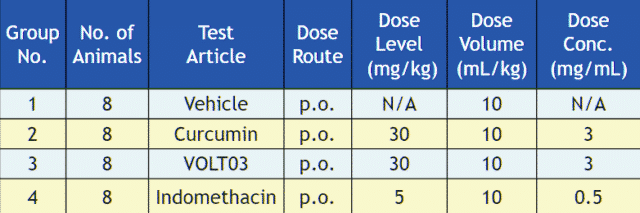
Methods: The paw was measured at 3 hours after oral gavage of curcumin (30 mg/kg), Indomethacin (5 mg/kg), and VOLT03 (30 mg/kg) to mice.
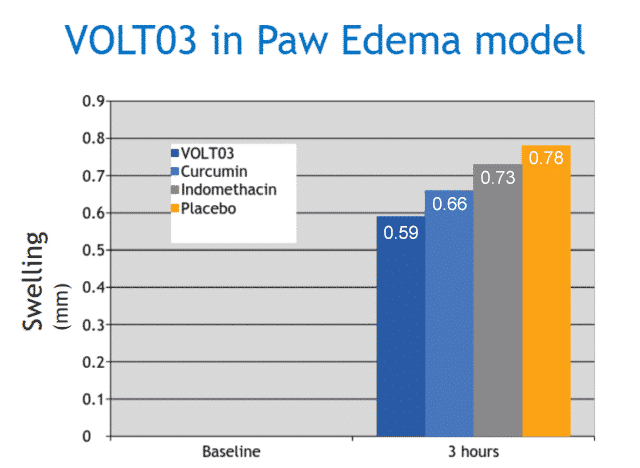
Results: After 3 hours, the swelling was 0.59 mm for VOLT03, 0.66 mm for curcumin, 0.73 mm for indomethacin, and 0.78 mm for vehicle control. The difference between VOLT03 and curcumin/indomethacin was statistically significant, with p<0.05.
PUBLICATIONS
- Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects
- A novel Curcumin Complex with Superior Efficacy to Indomethacin and Native Curcumin in an Inflammatory Model
- Volt03 shows significant anti-aging potential via enhancement of DNA repair mechanisms

ADVANTAGES OF VOLT03
- Only curcumin product to demonstrate statistically significant anti-inflammatory properties vs standard curcumin and an NSAID (indomethacin).
- Highly purified pharmaceutical-grade ingredients.
- Manufactured under cGMP in FDA-approved site.Ability to move quickly into OTC & international markets.
- Enhanced bioavailability allows for less frequent dosing.
- Highly soluble; thus final dosage form will be in a small vegetable capsule.
- Clean label – only cyclodextrin and curcumin in capsule.

